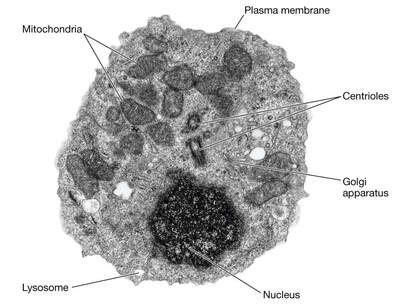
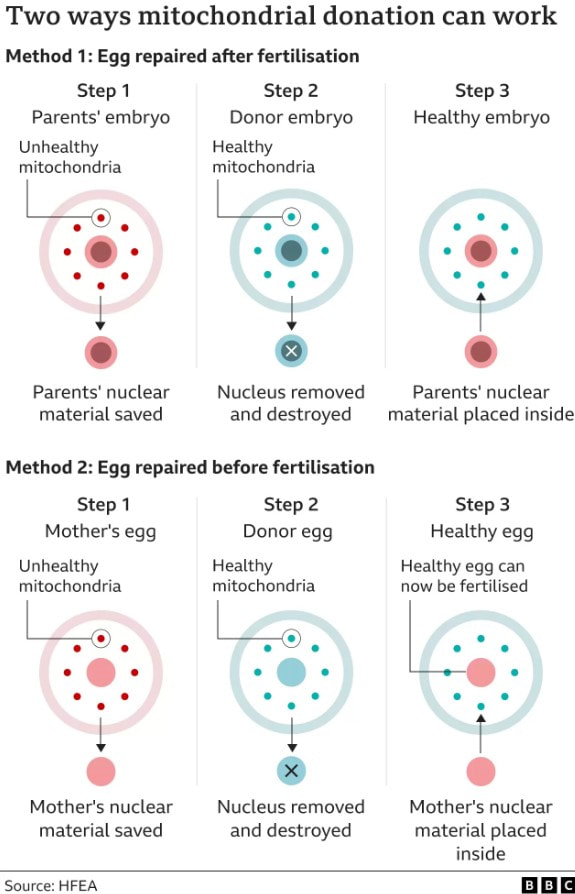
 Electron micrograph of a human lymphocyte. Credit: taken from hazell11bio.blogspot.com. Electron micrograph of a human lymphocyte. Credit: taken from hazell11bio.blogspot.com. John writes … Cells and organelles In order to comment on that headline, I need to start with a spot of cell biology. In all ‘eukaryotic’ organisms (i.e., all organisms except bacteria and archaea), cells contain within them several subcellular compartments or organelles. Two of these organelles, the mitochondrion (plural, mitochondria) and in plants, the chloroplast, contain a small number of genes, a reminder of their evolutionary past. These organelles were originally endosymbiotic within cells of the earliest eukaryotes. During subsequent evolution, the bulk of the endosymbionts’ genes have been taken over by the host’s main genome in the nucleus, leaving just a small proportion of their original genomes in the organelles. In mammals for example, the genes in the mitochondria make up about 0.0018 (0.18%) of the total number of genes. 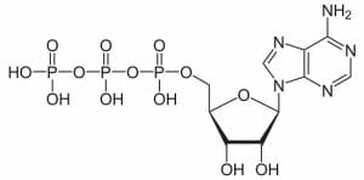 Diagram of ATP molecule. Diagram of ATP molecule. When mitochondria go wrong The main function of mitochondria is to convert the energy from food into a chemical energy currency called ATP. The average human cell makes and uses 150 million ATP molecules per day, emphasizing the absolutely key role that mitochondria play in the life of eukaryotes. This brings us back to mitochondrial genes. Although few in number, they are essential for mitochondrial function. Mutations in mitochondrial genes may lead to a lethal loss of mitochondrial function or may, in less severe cases, cause some impairment in function. During my time at the University of Exeter, I had direct contact with one such case. A very able student had elected to undertake his third-year research project with me and a colleague. However, towards the end of his second year his eyesight began to fail such that, by the start of his third year his vision was so impaired that a lab-based project was impossible. In a matter of a few months his eyesight had declined to about 15% of what he started with. The cause was a mitochondrial genetic mutation affecting the energising of the retina and hence causing impaired vision (2), a condition known as Leber hereditary optic neuropathy, symptoms of which typically appear in young adults. I need to say serious though it was for the student, this is one of milder diseases caused by mutations in a mitochondrial gene; many have much more serious effects (3), although I also need to say that diseases/syndromes based on mitochondrial genes are very rare. Is it GM or isn’t it? This brings us back to the BBC’s headline. Mitochondrial genes are only inherited from the mother. In fertilisation, the sperm delivers its full complement of nuclear genes to the egg cell but its mitochondria do not feature in the subsequent development of the embryo. In the early years of this century, scientists in several countries developed methods for replacing ‘faulty’ mitochondria in an egg cell or in a fertilised egg (one-cell embryo) with healthy mitochondria. This provides a way for a woman who knows she carries a mitochondrial mutation to avoid passing on that mutation to her offspring. However, there is another issue to deal with here. The replacement of one set of mitochondrial genes with another is clearly an example of genetic modification (GM), albeit an unusual example. In the UK, the Human Fertilisation and Embryology Act, whilst allowing spare embryos to be used in experimental procedures involving GM, prohibited the implantation of GM embryos to start a pregnancy. This would include embryos with ‘swapped’ mitochondrial DNA (or embryos derived from egg cells with swapped mitochondrial DNA). In order to bring this procedure into clinical practice, the law had to be changed which duly happened in 2015. Prior to the debates that led to the change in law, pioneers of the technique gave several presentations to explain what was involved; indeed, I was privileged to attend one of those presentations addressed to the bioscience and medical science communities. It was during this time that some opposition to the technique became apparent and the phrases ‘three-parent IVF’ and ‘three-parent babies’ became widespread both among opponents of the procedure and in the print and broadcast media.  Credit: John Bryant. Credit: John Bryant. Should we, or shouldn’t we? I will come back to the opposition in a moment but before that I want to ask whether our readers think that the use of the term ‘three-parent’ is justifiable. My friend, co-author and fellow-Christian, Linda la Velle and I discuss this on pages 52 to 54 of Introduction to Bioethics. In our view, the phrase is inaccurate and misleading and I was pleased to see that the recent BBC headline (in the title of this post) did not use it, even if the headline still conveyed slightly the wrong impression.  ‘Designer baby’. Credit: taken from www.makeuseof.com. ‘Designer baby’. Credit: taken from www.makeuseof.com. Returning to consider the opposition to the technique, there were some who believed that allowing it would open the gate to wider use of GM techniques with human embryos, leading to the possibility of ‘designer babies’. However, there were other reasons for opposition. Since its inception with the birth of Louise Brown in 1978, IVF has had its opponents who believe that it debases the moral status of the human embryo. According to their view, which I need to say, is not widely held (4), the one-cell human embryo should be granted the same moral status as a born human person, from the ‘moment of conception’. There can be no ‘spare’ embryos because that would be like saying there are spare people. This brings us to the two different techniques described in the recent BBC article. How is it done? As hinted at briefly already, there are two methods for removing faulty mitochondria and replacing them with fully functioning mitochondria, as shown in the HFEA diagram (below) reproduced by the BBC. In passing, I note that the ability to carry out these techniques owes a lot to what was learned during the development of mammalian cloning. In the first technique, an egg (5) is donated by a woman who has normal mitochondria. This egg is fertilised by IVF as is an egg from the prospective mother who is at risk of passing on faulty mitochondria. We now have two fertilised eggs/one-cell embryos. The nucleus (which contains most of the genes) is removed from the embryos derived from the donated egg and is replaced with the nucleus from the embryo with faulty mitochondria. Thus nuclear transfer has been achieved, setting up a ‘hybrid’ embryo (nucleus from the embryo with faulty mitochondria, ‘good’ mitochondria in the embryo derived from the donated egg). The embryo can then be grown on for two or three days before implantation into the prospective mother’s uterus, hopefully to start a pregnancy. However, readers will immediately realise that this method effectively involves destruction of a human one-cell embryo, raising again objections from those who hold the view that the early embryo has the same moral status as a person (see above). This brings us to the second method. It again involves donation of an egg with healthy mitochondria but this is enucleated without being fertilised. Nuclear transfer from an egg of the prospective mother with faulty mitochondria then creates a ‘hybrid’ egg which is only then fertilised by IVF and cultured for a few days prior to implantation into the uterus of the prospective mother. The technique does not inherently involve destruction of a one-cell human embryo. However, since the procedure, including IVF, will be carried out with several eggs, there still remains the question of spare embryos, mentioned above. Further, since this technique leads to less success in establishing pregnancies than the first technique, it is likely not to be the technique of first choice in these situations. So when was that, exactly? The BBC report which stimulated me to write this blog talked of a ‘UK First’ but that cannot mean that the world’s first case was in the UK. There are well-documented reports from at least three different countries and going back to 2016, of babies born following use of nuclear transfer techniques. The headline must imply that this was the first case in the UK. However, following the change in the law (mentioned above), in 2017 the Human Fertilisation and Embryology Authority (HFEA) licensed the Newcastle Fertility Centre to carry out this procedure. It was predicted that the first nuclear transfer/mitochondrial donation baby would be born in 2018 (although we emphasise that because of the rarity of these mitochondrial gene disorders the number of births achieved by this route will be small). So, when was the first such baby actually born in the UK. The answer is that we do not know. The HFEA, which regulates the procedure, is very protective of patient anonymity and does not release specific information that might identify patients. All we know is that ‘fewer than five’ nuclear transfer/mitochondrial donation babies have been born and that the births have taken place between 2018 and early 2023 – so, in respect of the date of this ‘UK First’ we are none the wiser. John Bryant
Topsham, Devon 28 June 2023 (1) Baby born from three people’s DNA – BBC News. (2) My colleague and I were able to offer him a computer-based project and the university’s special needs office provided all that he needed to complete his degree. (3) See, for example: Mitochondrial DNA Common Mutation Syndromes, Children’s Hospital of Philadelphia – chop.edu. (4) In Life in Our Hands (IVP, 2004) and Beyond Human? (Lion Hudson, 2012), I discuss the wide range of views on this topic among Christians. (5) Although I use the singular for the sake of convenience, several donated and several maternal eggs are used in these procedures.
0 Comments
Leave a Reply. |
AuthorsJohn Bryant and Graham Swinerd comment on biology, physics and faith. Archives
July 2024
Categories |
 RSS Feed
RSS Feed
